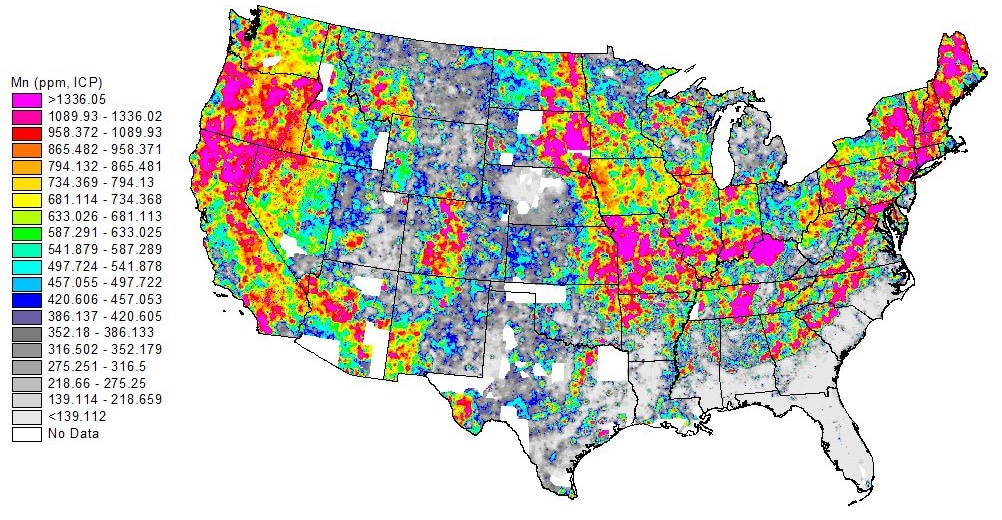
Manganese in drinking water has recently come under scrutiny due to its potential toxicity as well as its damage to distribution systems. A mineral similar to iron and common in Earth’s crust, manganese is found in about 95% of New England water supplies. While low concentrations are not only safe but also beneficial to human health, elevated manganese concentrations can cause taste and color issues, health risks to customers, and problems for distribution systems.
Health Effects of Manganese
 Manganese is an essential nutrient at about 2.5-5.0 mg/day, but overexposure can potentially cause serious health issues. Long term exposure to manganese can cause toxicity to the nervous system and Parkinson’s like symptoms – particularly in children, the elderly, and pregnant mothers. Young children and infants cannot break down manganese in their bodies as effectively as adults, which can cause issues in early brain development. In recent studies, children exposed to high levels of manganese experienced learning difficulties such as ADD, hyperactivity, Pervasive Development Disorder, and memory issues. Another interesting effect of overexposure to manganese is violent behavior. Studies have shown excessive manganese decreases serotonin function and reduces dopamine levels, resulting in social withdrawal, increased depression, and aggression. Studies completed in prisons have concluded manganese toxicity contributes to delinquent behavior, and autopsies of mass murderers often show toxic levels of manganese. While these studies may be concerning, manganese ingested through drinking water is processed by the liver and reduces the risks associated with other forms of manganese exposure, such as inhaling.
Manganese is an essential nutrient at about 2.5-5.0 mg/day, but overexposure can potentially cause serious health issues. Long term exposure to manganese can cause toxicity to the nervous system and Parkinson’s like symptoms – particularly in children, the elderly, and pregnant mothers. Young children and infants cannot break down manganese in their bodies as effectively as adults, which can cause issues in early brain development. In recent studies, children exposed to high levels of manganese experienced learning difficulties such as ADD, hyperactivity, Pervasive Development Disorder, and memory issues. Another interesting effect of overexposure to manganese is violent behavior. Studies have shown excessive manganese decreases serotonin function and reduces dopamine levels, resulting in social withdrawal, increased depression, and aggression. Studies completed in prisons have concluded manganese toxicity contributes to delinquent behavior, and autopsies of mass murderers often show toxic levels of manganese. While these studies may be concerning, manganese ingested through drinking water is processed by the liver and reduces the risks associated with other forms of manganese exposure, such as inhaling.
State and Federal Guidelines for Manganese

There are currently no enforceable federal drinking water standards for manganese. The US Environmental Protection Agency (EPA) has a secondary standard of 0.05 mg/L, a standard established to address issues of aesthetics such as discoloration, rather than health concerns. In the absence of an enforceable federal standard, the Connecticut Department of Public Health (CT DPH), has set their Action Level at 0.5 mg/L, whereas the Massachusetts Office of Research and Standards has set an Office of Research and Standards Guideline Limit (ORSGL) of 0.3 mg/L for lifetime exposure by adults and acute exposure (ten days) by infants less than one year of age.
Saving the Distribution System
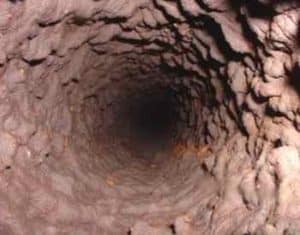 Manganese deposits can build up in pipelines, pressure tanks, water heaters, and water softeners, reducing the available quantity of the water supply and pressure in the system. Manganese accumulations can become expensive for utilities when water supply or water softening equipment must be replaced. Also, energy costs can become a burden for utilities when pumping water through constricted pipes or heating water with heating rods coated with manganese deposits. Managing safe levels of manganese in drinking water is an important step in preserving valuable assets in a distribution system. The benefits associated with treating manganese greatly outweigh the long-term repair and rehabilitation costs utilities may face with high levels of manganese. To adequately manage safe levels of manganese, proper water treatment is paramount.
Manganese deposits can build up in pipelines, pressure tanks, water heaters, and water softeners, reducing the available quantity of the water supply and pressure in the system. Manganese accumulations can become expensive for utilities when water supply or water softening equipment must be replaced. Also, energy costs can become a burden for utilities when pumping water through constricted pipes or heating water with heating rods coated with manganese deposits. Managing safe levels of manganese in drinking water is an important step in preserving valuable assets in a distribution system. The benefits associated with treating manganese greatly outweigh the long-term repair and rehabilitation costs utilities may face with high levels of manganese. To adequately manage safe levels of manganese, proper water treatment is paramount.
Proper Testing
For managing manganese in drinking water, the best treatment method is dependent on several factors including manganese concentrations, the presence of other contaminants, and existing treatment methods. Therefore, accurate testing is important before considering options or selecting treatment equipment. Typically, tests are conducted to quantify the extent of manganese concentrations, but testing of additional water parameters such as pH, oxygen content, hardness, iron, and sulfur may also be useful to determine the most appropriate water treatment method.
Phosphate Treatment
 For low concentrations of manganese, 0.3 mg/L or less, sequestering utilizing phosphate compounds is a simple, effective, and inexpensive solution. When added to water, phosphate compounds surround minerals and keep them in solution. When these compounds are put into the water system, they stabilize and disperse dissolved manganese. As a result, the manganese is not available to react with oxygen to create issues with the color, taste, or odor of drinking water. The phosphate compounds must be introduced into the water at a point where the manganese is still dissolved to maintain water clarity. This treatment process should take place before the pressure tank and as close to the well discharge point as possible. Phosphate treatment does come with a bit of risk due to the instability of most phosphate compounds at higher temperatures. If phosphate-treated water is boiled or heated, such as in a water heater, the compounds have the potential to break down and release manganese that could react with oxygen and precipitate. Also, phosphates from any source contribute to excess nutrient content in surface water.
For low concentrations of manganese, 0.3 mg/L or less, sequestering utilizing phosphate compounds is a simple, effective, and inexpensive solution. When added to water, phosphate compounds surround minerals and keep them in solution. When these compounds are put into the water system, they stabilize and disperse dissolved manganese. As a result, the manganese is not available to react with oxygen to create issues with the color, taste, or odor of drinking water. The phosphate compounds must be introduced into the water at a point where the manganese is still dissolved to maintain water clarity. This treatment process should take place before the pressure tank and as close to the well discharge point as possible. Phosphate treatment does come with a bit of risk due to the instability of most phosphate compounds at higher temperatures. If phosphate-treated water is boiled or heated, such as in a water heater, the compounds have the potential to break down and release manganese that could react with oxygen and precipitate. Also, phosphates from any source contribute to excess nutrient content in surface water.
Oxidation Followed by Filtration

Among the most common forms of manganese treatment is oxidation followed by filtration. This form of treatment is ideal for manganese concentrations greater than 0.3 mg/L, where sequestering is not an option. During this process, an oxidizing chemical, often potassium permanganate, chlorine, or ozone, is pumped into the water by a small chemical metering pump that operates simultaneously with the well pump. This step converts soluble manganese into an insoluble, filterable form. Typically, the chemical is injected in a pipeline prior to the filters, providing sufficient contact time to allow oxidation to take place. The resulting solid particles then must be filtered. Therefore, a media, membrane, or biological filter is necessary for the removal process. Common media filters include GreensandPlus and LayneOx®; membrane filtration technologies include microfiltration, ultrafiltration, and nanofiltration; and biological filtration technologies include Mangazur®. While the process may seem simple, it is important to monitor both the source water and treated water to determine the proper oxidation dosage and confirm the removal efficiency.
In Conclusion
When managing manganese levels in drinking water, it is imperative to have a well-executed balance between maximizing quality while minimizing costs. While there are many different methods to treat manganese in drinking water, the best first step to take is proper testing and an evaluation of the distribution system. Every system is different and may require unique treatment or even new source development. Manganese poses a problem for both communities and utilities alike, and proper mitigation protects the health of water system customers while greatly increasing the condition and life of the water distribution system.
 Ryan Neyland, P.E. Project Manager, has over 11 years of concentrated water treatment experience including all phases of planning, design, and construction services, as well as pump station rehabilitation and SCADA experience. He holds a BS in Civil Engineering from Worcester Polytechnic Institute.
Ryan Neyland, P.E. Project Manager, has over 11 years of concentrated water treatment experience including all phases of planning, design, and construction services, as well as pump station rehabilitation and SCADA experience. He holds a BS in Civil Engineering from Worcester Polytechnic Institute.
